



Category Number
MOQ
Customized Service
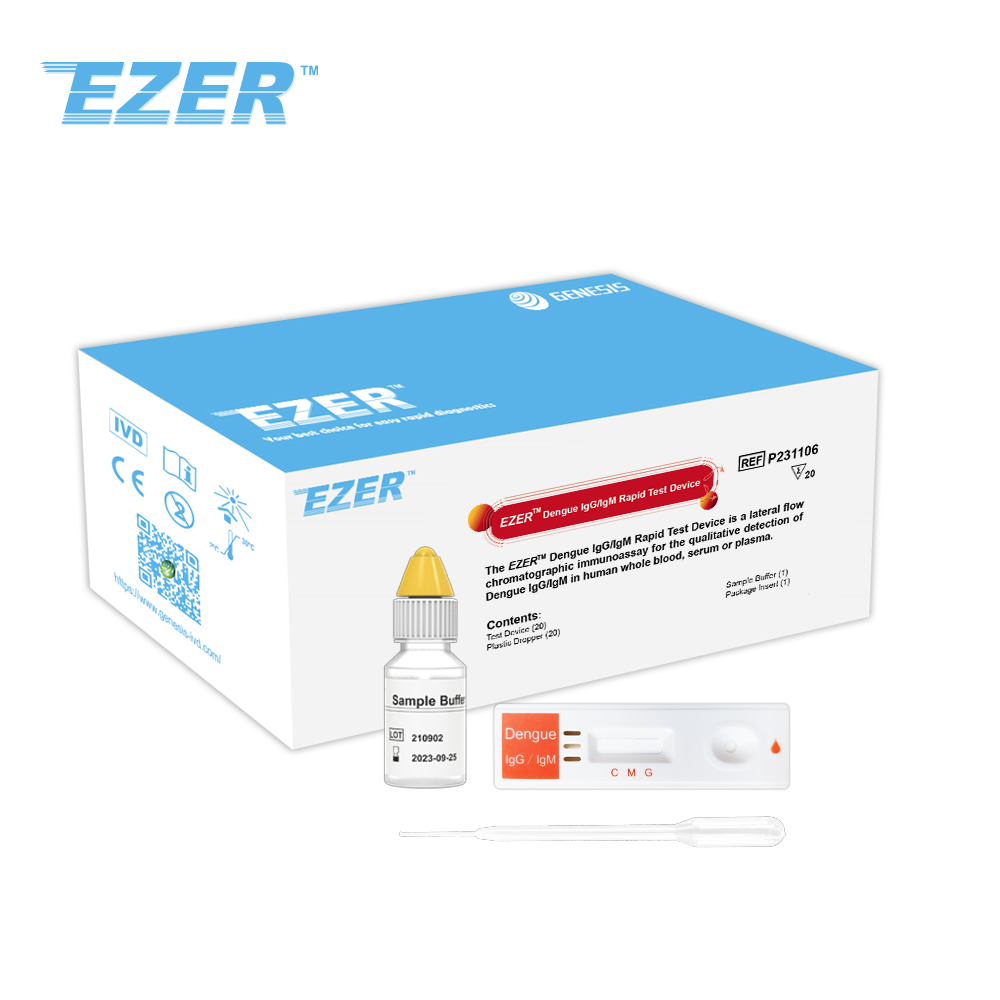
The EZER™ Dengue IgG/IgM Rapid Test Device is a lateral flow chromatographic immunoassay for the qualitative detection of dengue IgG and IgM in human whole blood, serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with Dengue viruses. Any reactive specimen with the Dengue IgG/IgM Rapid Test must be confirmed with alternative testing method(s) and clinical findings.
Human whole blood
Serum
Plasma
Allow the samples to react according to the procedure and read the lines that appear in the reading area.
All reagents are ready to use as supplied. Store unused test device unopened at 2°C-30°C.
The positive and negative controls should be kept at 2°C-8°C. If stored at 2°C-8°C, ensure that the test device is brought to room temperature before opening. The test device is stable through the expiration date printed on the sealed pouch.
Do not freeze the kit or expose the kit over 30°C.
|
Cat. No. |
P221104 |
|
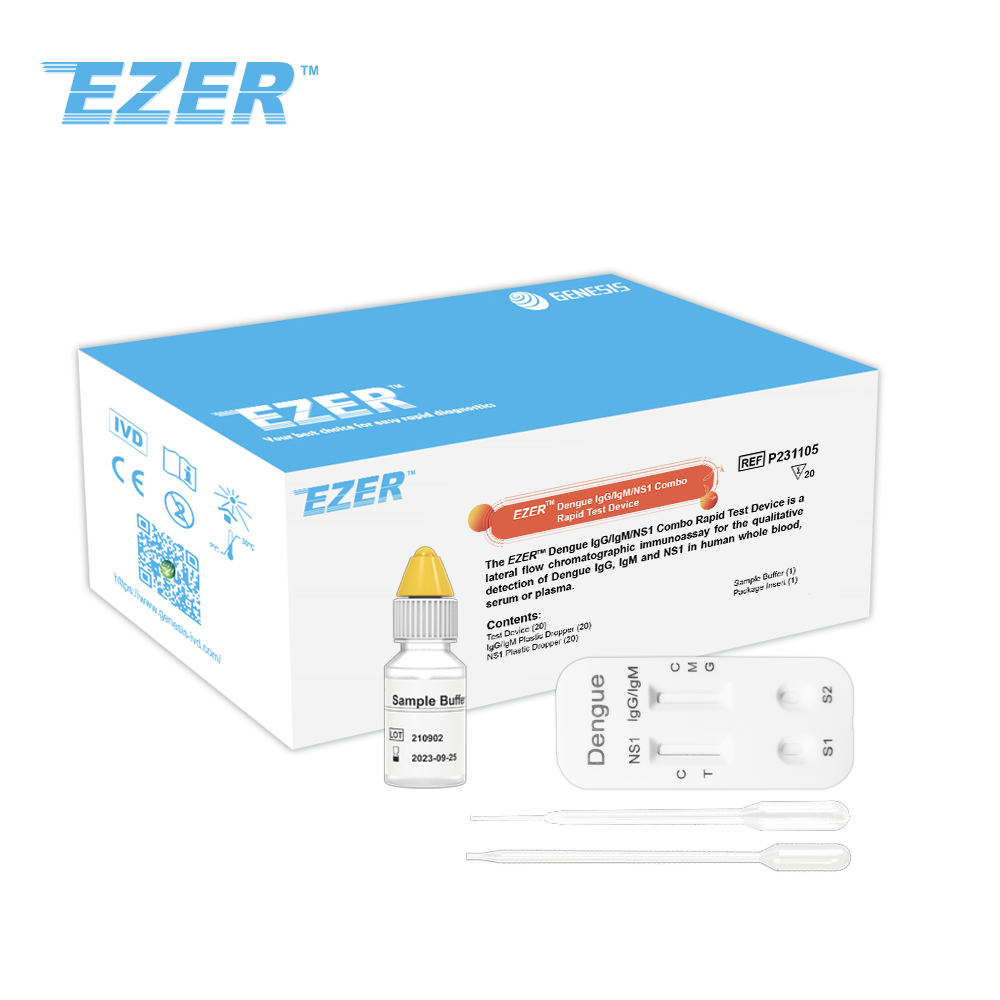
Dengue IgG/IgM test device |
20 |
|
Sample buffer, 4ml |
1 |
|
Plastic dropper |
20 |
|
Package insert |
1 |
|
Product |
Cat. No. |
Contents |
|
EZER™ Dengue IgG/IgM Rapid Test Device |
P231106 |
20 Tests |


1. what's the validity period?
The validity period is 24 months for the majority Genesis’ products.
The validity period of the products may vary depending on the product characteristics.
2. What's the lead time of your products?
Payment need to be made before the delivery. Normally delivery is made within one week after the payment.
3. What are the minimum order quantity(MOQ) for ordering?
The MOQ for ordering is 5,000 units. Customized alternative solutions will be available if your order below 5,000.