




Category Number
MOQ
Customized Service
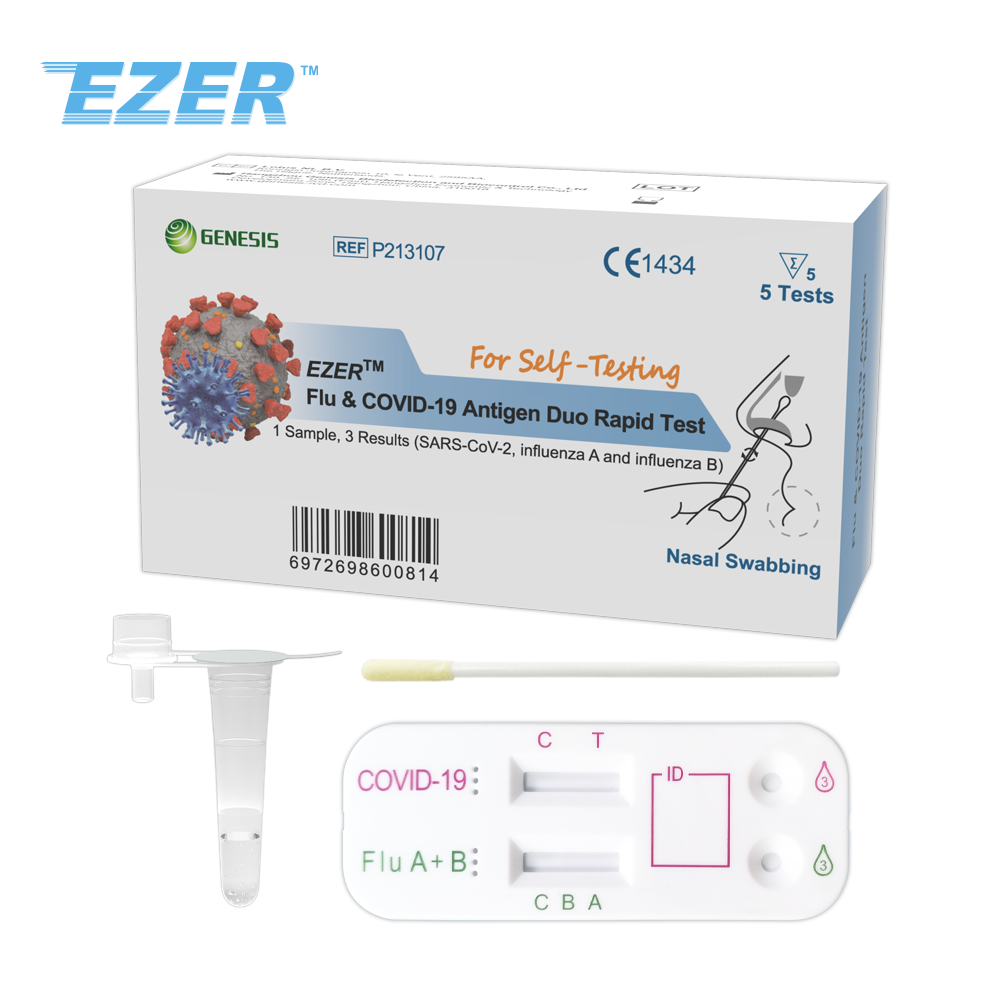
The EZER™ HPIVs Antigen Rapid Test Device is an in vitro diagnostic test for the qualitative detection of Human parainfluenza virus type 1, 2, 3 nucleoprotein antigens in nasopharyngeal (NP) swab and nasal aspirate samples, using the rapid immunochromatographic method.
The detection is based on the monoclonal antibodies specific for the nucleoprotein of either Human parainfluenza virus.
It is intended to aid in the rapid diagnosis of Human parainfluenza virus type 1, 2, 3 infection.
Nasopharyngeal Swabbing
Nasal Aspiration
Read results at 15 minutes and no more than 30 minutes.
Test devices must be stored at 2~30°C. DO NOT FREEZE.
|
Cat. No. |
P213114 |
|
Test device |
20 |
|
Sterilized swab |
20 |
|
Extraction tube(with o.5ml extraction buffer) |
20 |
|
Nozzle |
20 |
|
Tube Stand |
1 |
|
Package Insert |
1 |
|
Product |
Cat. No. |
Contents |
|
EZER™ HPIVs Antigen Rapid Test |
P213114 |
20 Tests |



1. what's the validity period?
The validity period is 24 months for the majority Genesis’ products.
The validity period of the products may vary depending on the product characteristics.
2. What's the lead time of your products?
Payment need to be made before the delivery. Normally delivery is made within one week after the payment.
3. What are the minimum order quantity(MOQ) for ordering?
The MOQ for ordering is 5,000 units. Customized alternative solutions will be available if your order below 5,000.