




Category Number
MOQ
Customized Service
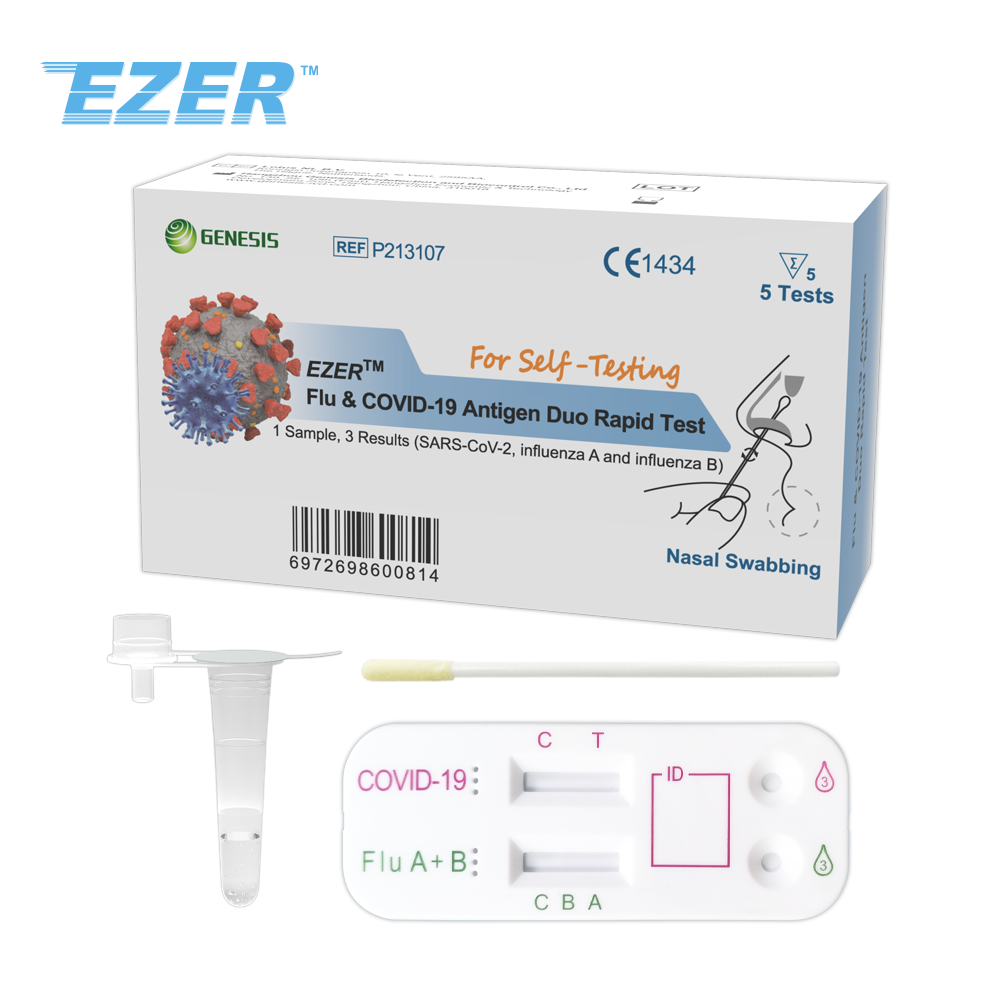
The EZER™ SARS-CoV-2 & Flu & RSV & ADV Antigen Combo Rapid Test Device is intended for the simultaneous qualitative detection and differentiation of the nucleocapsid protein antigens from SARS-CoV-2, influenza A, influenza B, Respiratory Syncytial Virus and Adenovirus in direct nasal or nasopharyngeal swab specimens. It’s an in vitro diagnostic test for professional use only.
The detection is based on the antibodies which were developed specifically recognizing and reacting with the nucleoprotein of virus.
Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2, influenza, RSV and ADV can be similar. SARS-CoV-2, influenza A, influenza B, RSV and ADV viral antigens are generally detectable in upper respiratory specimens during the acute phase of infection.
Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status.
Nasal Swabbing
Nasopharyngeal Swabbing
|
Cat. No. |
P211145 |
|
Test devices |
20 |
|
Sterilized swabs |
20 |
|
Extraction tubes |
20 |
|
Nozzles |
20 |
|
Tube Stand |
1 |
|
Package insert |
1 |
|
Product |
Cat. No. |
Contents |
|
EZER™ SARS-CoV-2 & Flu & RSV & ADV Antigen Combo Rapid Test Device |
P211145 |
20 Tests |



1. what's the validity period?
The validity period is 24 months for the majority Genesis’ products.
The validity period of the products may vary depending on the product characteristics.
2. What's the lead time of your products?
Payment need to be made before the delivery. Normally delivery is made within one week after the payment.
3. What are the minimum order quantity(MOQ) for ordering?
The MOQ for ordering is 5,000 units. Customized alternative solutions will be available if your order below 5,000.