



Category Number
MOQ
Customized Service
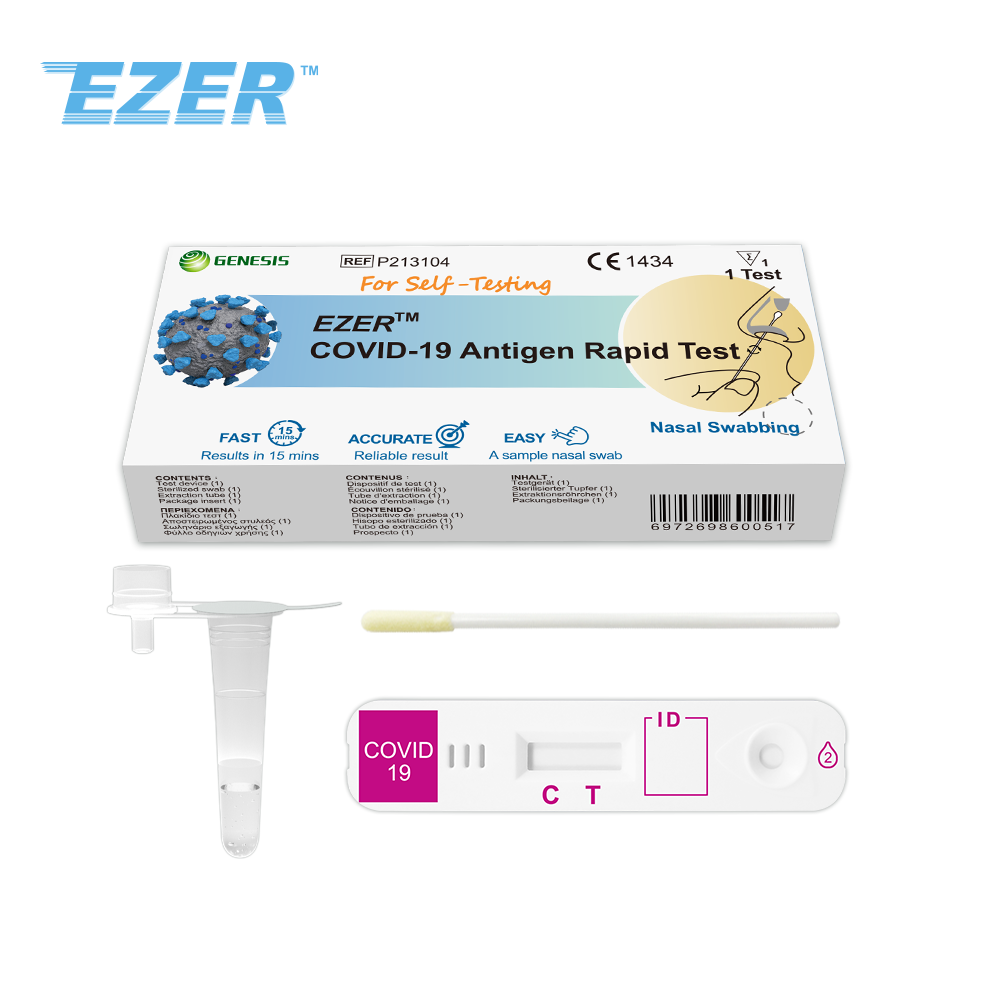
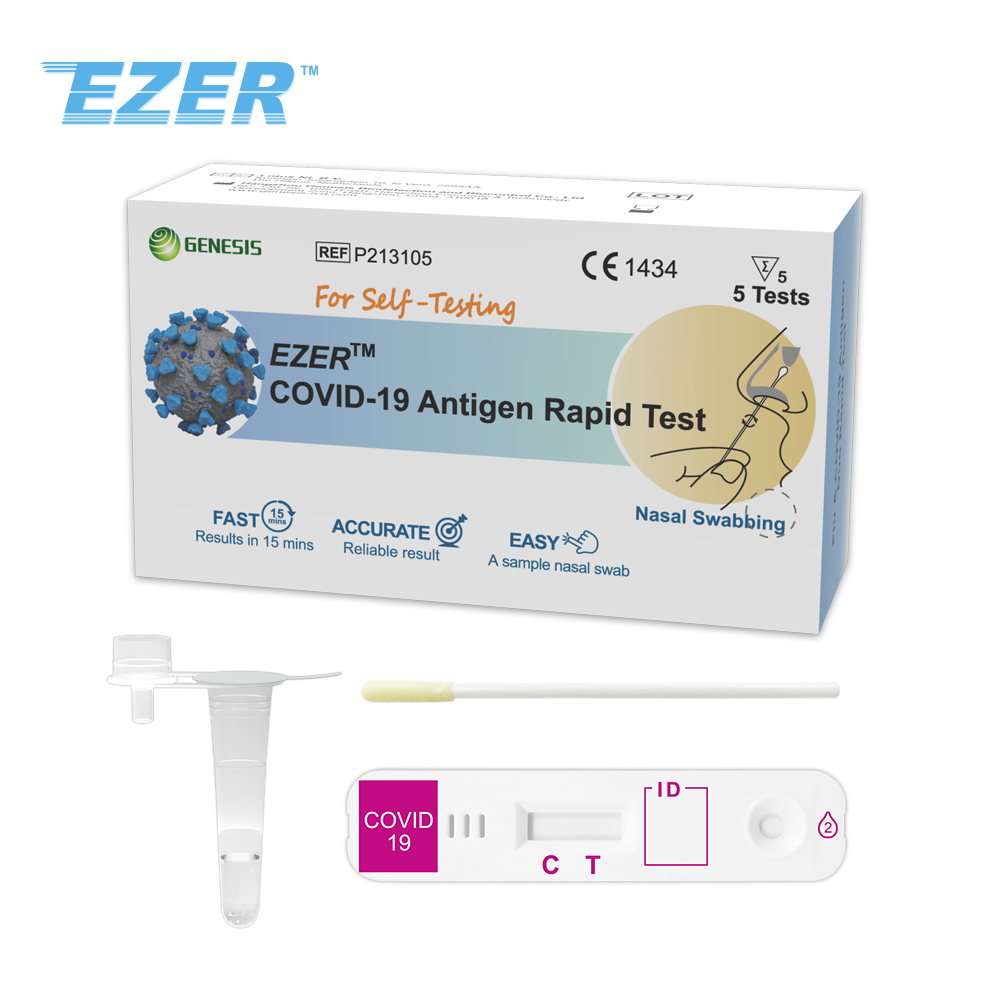
The EZER™ COVID-19 Antigen Rapid Test is a rapid, lateral flow immunochromatography assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal swab specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 infection. This test is for laymen with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older, or adult collected anterior nasal swab samples from individuals aged 2 years or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
The EZER™ COVID-19 Antigen Rapid Test does not differentiate between SARSCoV and SARS-CoV-2. Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in anterior nasal (nares) swabs during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Individuals who test positive should self-isolate and consult with their healthcare provider as additional testing may be necessary and for public health reporting. Negative results should be treated as presumptive, do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of an individual’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
Nasal Swabbing
Read results at 15 minutes and no more than 30 minutes.
Test devices must be stored at 2~30°C. DO NOT FREEZE. After storage in refrigerator, devices must be brought back to room temperature for at least 30 minutes before testing.
|
Cat. No. |
P213104 |
P213105 |
P213108 |
|
Test device |
1 |
5 |
20 |
|
Sterilized swab |
1 |
5 |
20 |
|
Extraction tube (with Extraction buffer) |
1 |
5 |
20 |
| Tube Stand |
|
|
1 |
|
Package Insert |
1 |
1 |
1 |
|
Product |
Cat. No. |
Contents |
|
EZER™ COVID-19 Antigen Rapid Test Device |
P213104 |
1 Test |
|
P213105 |
5 Tests |
|
|
P213108 |
20 Tests |



1. what's the validity period?
The validity period is 24 months for the majority Genesis’ products.
The validity period of the products may vary depending on the product characteristics.
2. What's the lead time of your products?
Payment need to be made before the delivery. Normally delivery is made within one week after the payment.
3. What are the minimum order quantity(MOQ) for ordering?
The MOQ for ordering is 5,000 units. Customized alternative solutions will be available if your order below 5,000.